Principles of Photocatalyst

Photocatalyst, as the name implies, is the substance which will perform a catalyst function after absorbing the energy of light. Early in 1930s, there were documents about Photocatal-yst in which ZnO plays the main role of reaction, but semiconductor TiO2 has been used to make Photo-catalyst since Fujishima Akira, a Japanese scientist, discovered the my-sterious features of TiO2 in 1968. Currently, the academc definition of Photocatalyst is “the semiconductor applying mat-erial, which is composed of anatase n-type semicondu-ctor TiO2”. There is a wide variety of Photocatalyst’s application and commercially it is used in the form of liquid by and large.
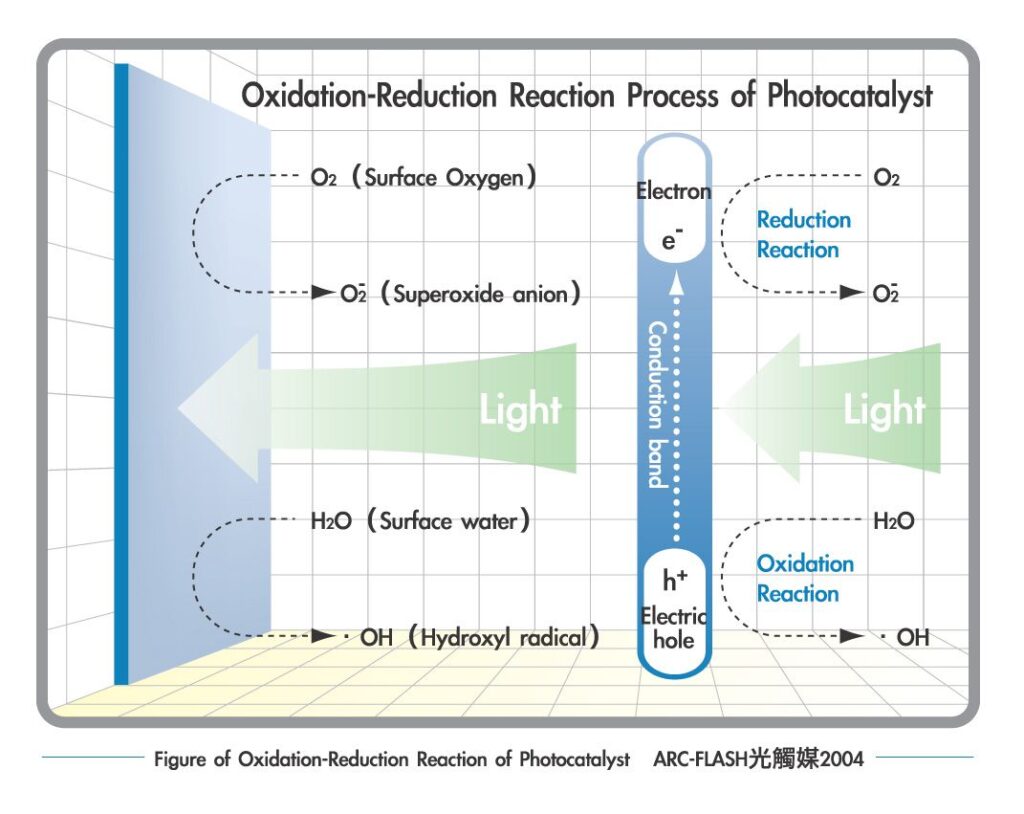
Photocatalyst Fundamentality
Once light hits Photocatalyst, the electron in TiO2 will jump from the valence band to the conducti-on band, and electron (e-) and electric hole (h+) pairs will form on the surface of Photo-catalyst. The negative electrons and oxygen will combine into O2-; the positive electric holes and water will generate hydroxyl radicals. Since both are unstable chemical substances, when the organic compound falls on the surface of Photocatalyst it will combine with O2- and OH- respectively and turn into dioxide carbon (CO2) and water (H2O). This cascade reaction is called “Oxidation-Reduction”.
Through the reaction, Photocatalyst is able to decompose common organic matters in the air, such as odor molecules, bacteria and virus to help decontaminate indoor environment and purify the air. Therefore, in the latest 10 years, Photocatalyst has been widely applied in cleaning of houses and medical institutions in Japan. Additionally, the fabric products processed by Photocatalyst have become a new option for hospital or clinical usages.

